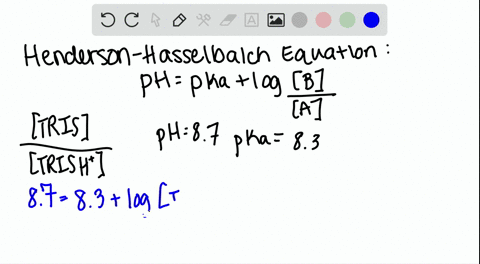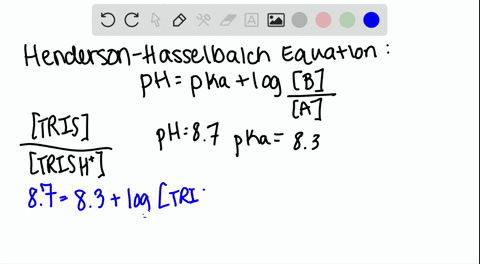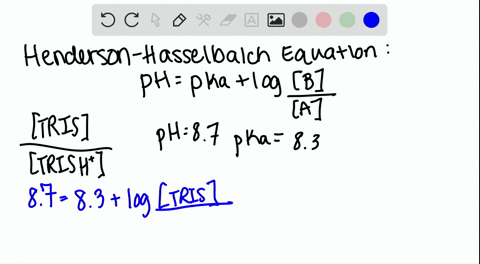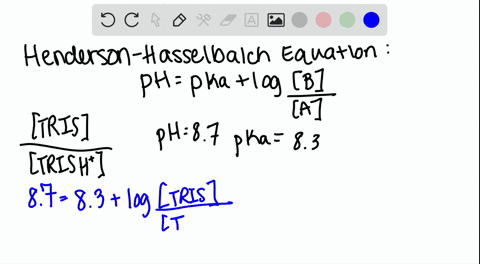
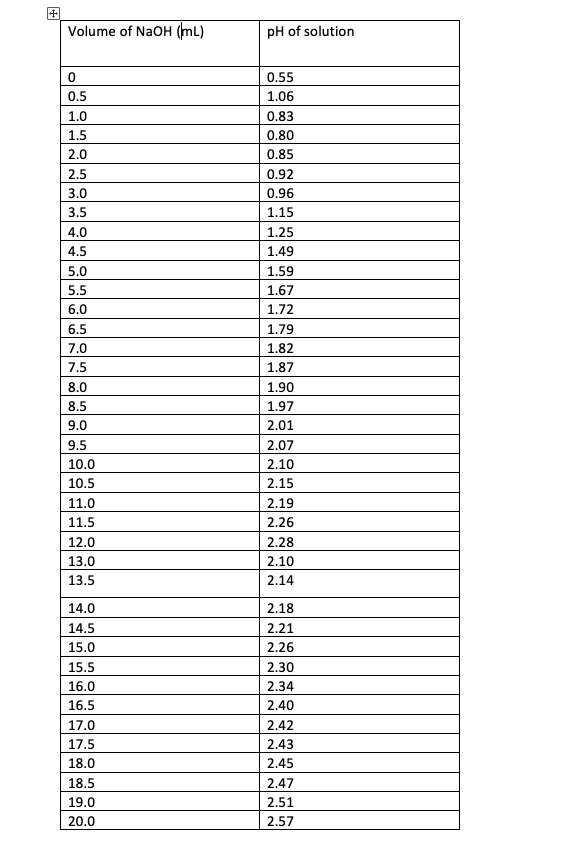
Dissociation steps and pKa values at 25 °C and 37 °C of the buffers... | Download Scientific Diagram
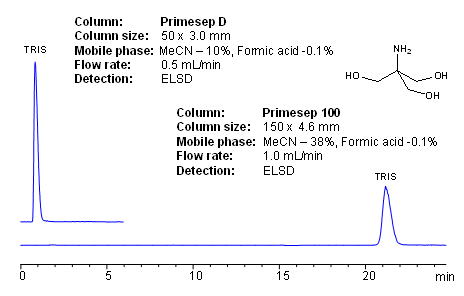
HPLC Method for Analysis of Trometamol (Tris, Tris(hydroxymethyl)aminomethane, Tromethamine, and or THAM) | SIELC Technologies
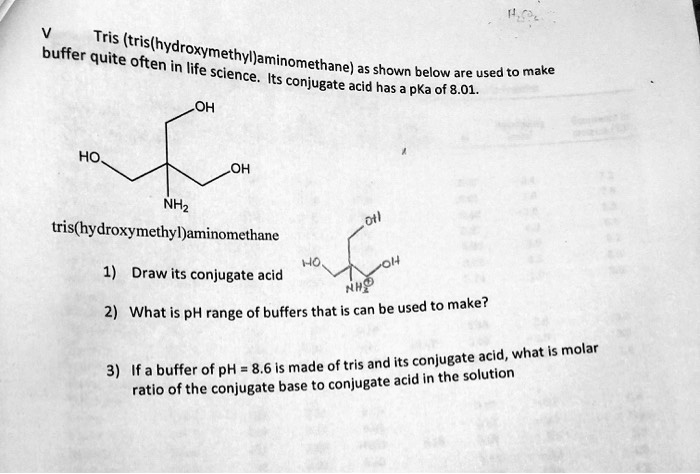
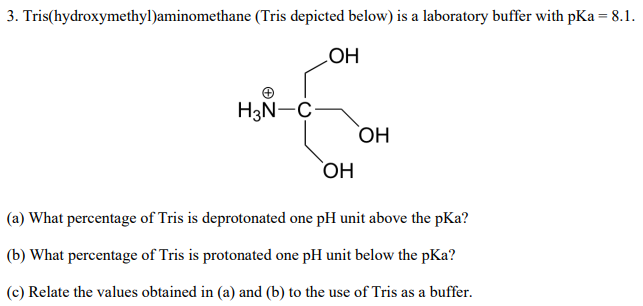
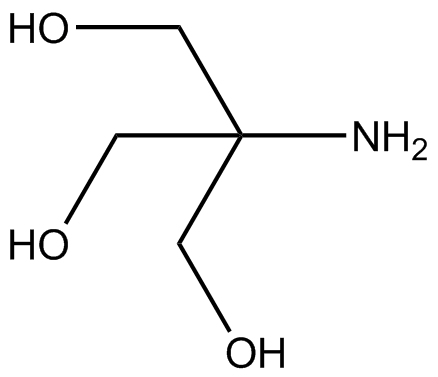
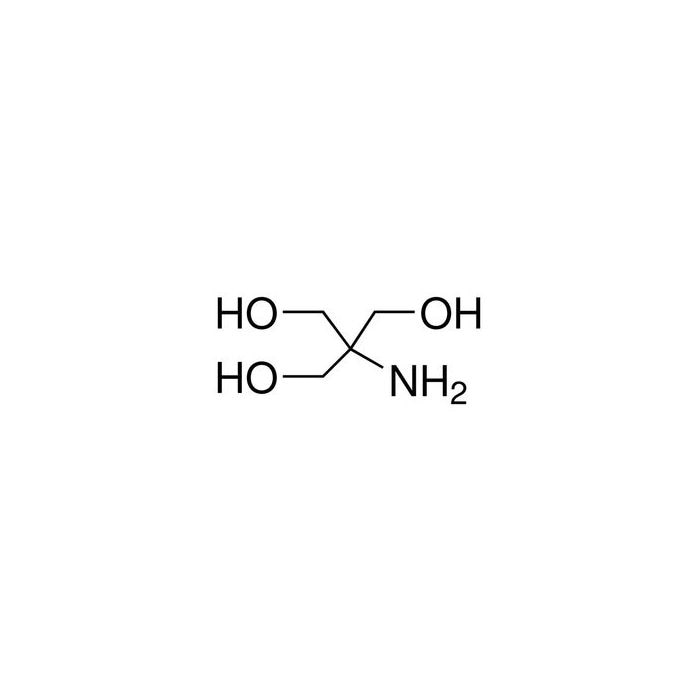
SOLVED: Tris buffer is quite often used in life science. Tris(hydroxymethyl)aminomethane is a common buffer that has a conjugate acid with a pKa of 8.01. The structure of tris(hydroxymethyl)aminomethane is shown below:
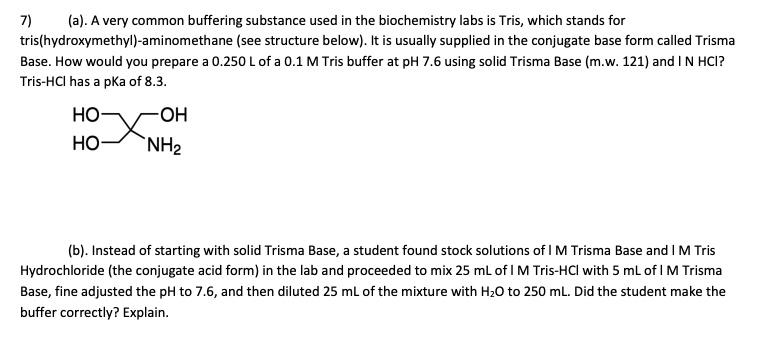
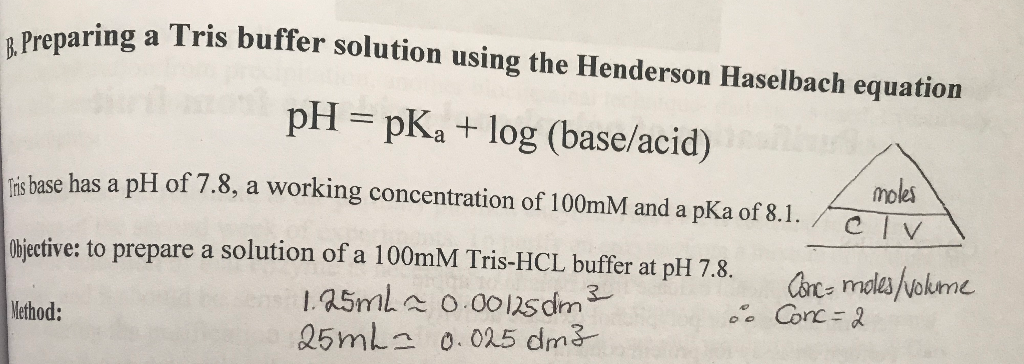
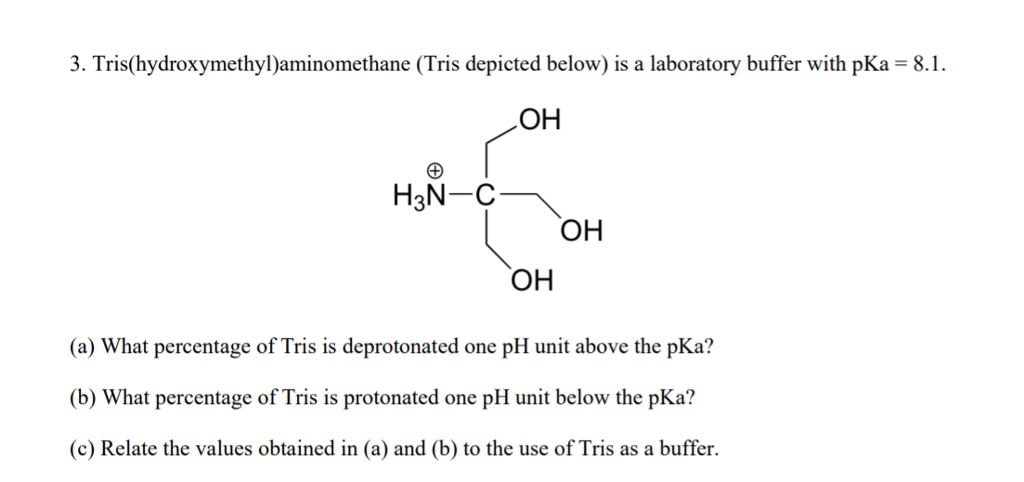
SOLVED: (a) A very common buffering substance used in biochemistry labs is Tris, which stands for tris(hydroxymethyl)aminomethane (see structure below). It is usually supplied in the conjugate base form called Trisma Base .


![T60040-5000.0 - Tris Base Ultra Pure [Tris (Hydroxymethyl) Aminomethane], 5 Kilograms T60040-5000.0 - Tris Base Ultra Pure [Tris (Hydroxymethyl) Aminomethane], 5 Kilograms](https://d2gdaxkudte5p.cloudfront.net/system/images/plabel_14934_20220328-164223.jpg)