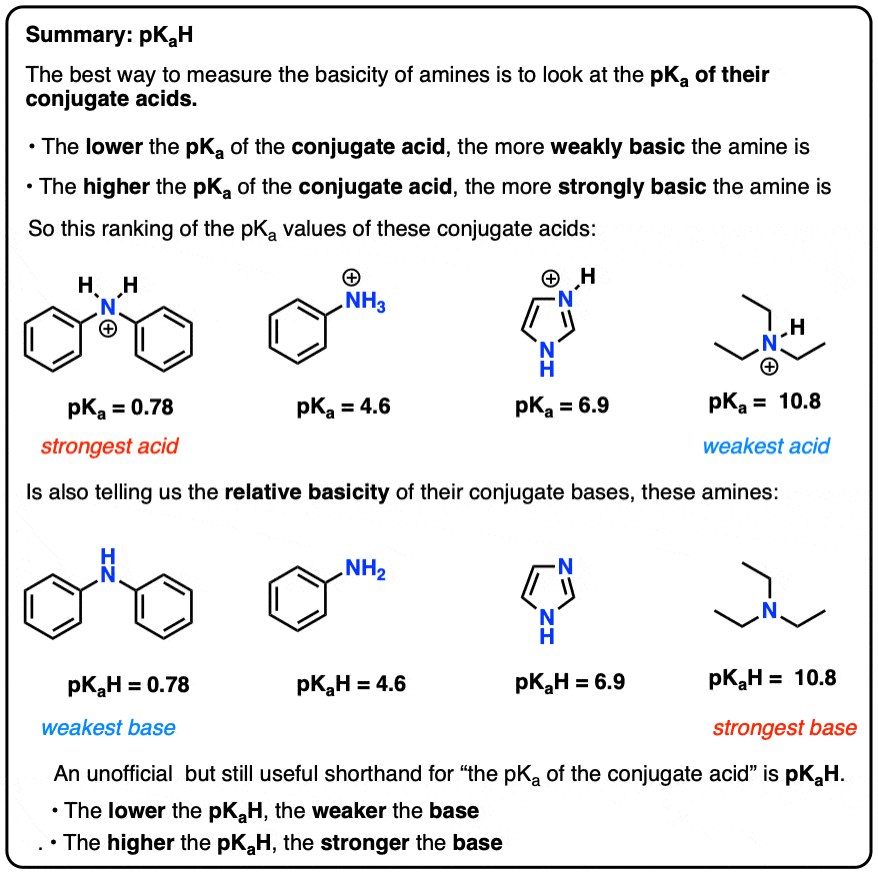
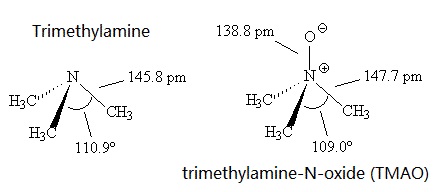
mass spectrum of N,N-dimethylmethanamine (trimethylamine) C3H9N (CH3)3N fragmentation pattern of m/z m/e
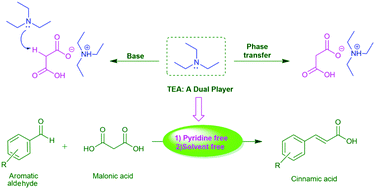
Triethylamine: a potential N-base surrogate for pyridine in Knoevenagel condensation of aromatic aldehydes and malonic acid - New Journal of Chemistry (RSC Publishing)

18856-86-5 | Trimethylamine-d9 Hydrochloride | N,N-Dimethylmethanamine-d9 Hydrochloride; | C₃D₉N • HCl | TRC
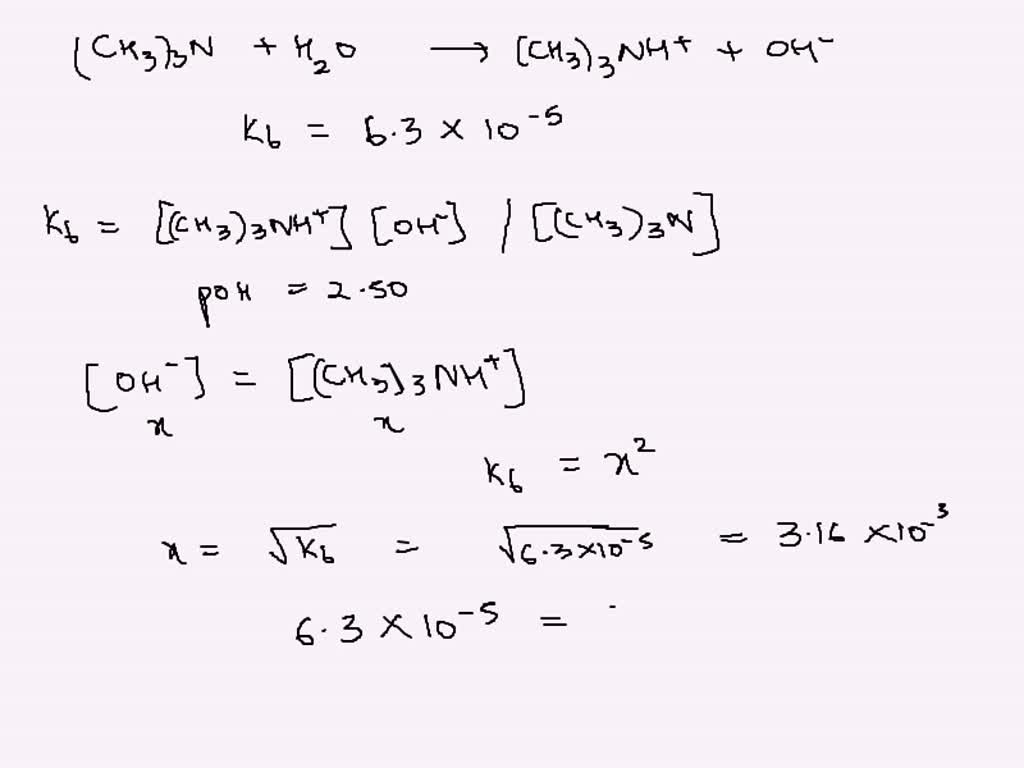
SOLVED: Trimethylamine, (CH3)3N, is a weak base (Kb = 6.3 × 10–5). What volume of this gas, measured at STP, must be dissolved in 2.5 L of solution to give that solution

SOLVED: 'The compound trimethylamine, (CH3)3N, is a weak base when dissolved in water Write the Kb expression for the weak base equilibrium that occurs in an aqueous solution of trimethylamine: Kb'
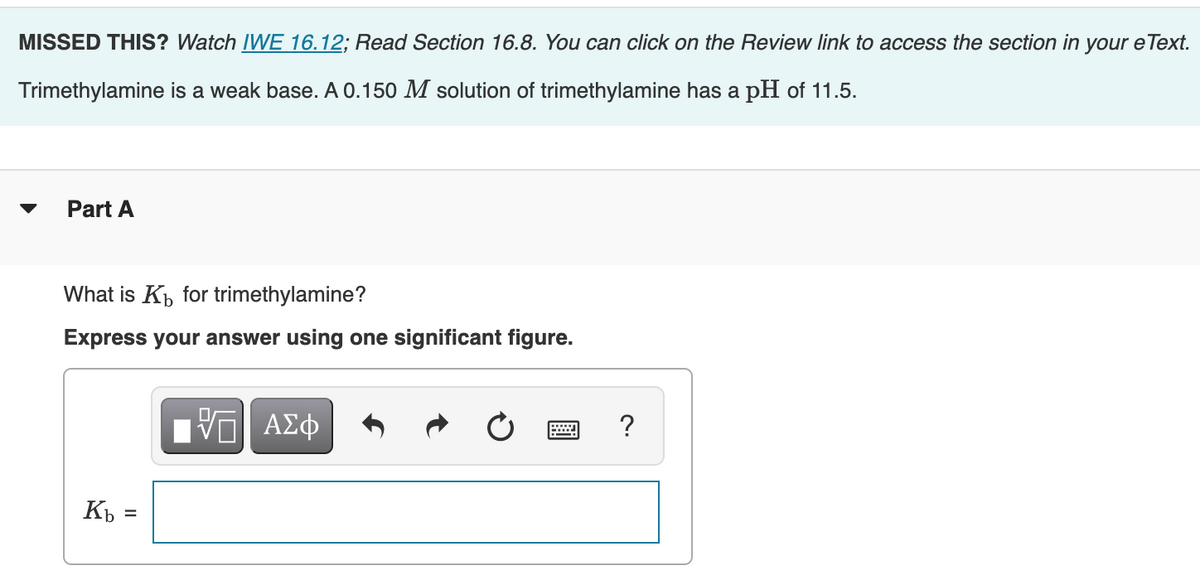
✓ Solved: Base Ionization Trimethylamine, (CH3)3N, is a gas with a fishy, ammonialike odor. An aqueous...

Pls explain: Q Trisilyl amine is a weaker base than trimethyl amine because (a) in trisilyl amine pπ-pπ bonding - Chemistry - The p-Block Elements - 12586833 | Meritnation.com

Trimethylamine Is A Nitrogenous Base And Can Be Protonated To Trimethylammonium Cation. This Colorless, Hygroscopic, And Flammable Tertiary Amine Has A Strong Fishy Or An Ammonia-like Odor Stock Photo, Picture And Royalty

Calculate the pH of a 0.443 M aqueous solution of trimethylamine. (Kb = 6.3 x 10-5) | Homework.Study.com