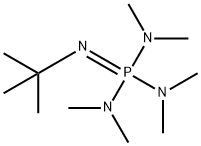
![2-tert-Butylamino-1-methyl-2-[tris(dimethylamino)phosphoranylidenamino]-perhydro-1,3,2-diazaphosphorinium iodide purum, ≥97.0% (CHN) | Sigma-Aldrich 2-tert-Butylamino-1-methyl-2-[tris(dimethylamino)phosphoranylidenamino]-perhydro-1,3,2-diazaphosphorinium iodide purum, ≥97.0% (CHN) | Sigma-Aldrich](https://www.sigmaaldrich.com/deepweb/content/dam/sigma-aldrich/structure5/127/mfcd01863108.eps/_jcr_content/renditions/mfcd01863108-medium.png)
2-tert-Butylamino-1-methyl-2-[tris(dimethylamino)phosphoranylidenamino]-perhydro-1,3,2-diazaphosphorinium iodide purum, ≥97.0% (CHN) | Sigma-Aldrich

Anionic ring‐opening polymerization of N‐glycidylphthalimide: Combination of phosphazene base and activated monomer mechanism - Rassou - 2018 - Journal of Polymer Science Part A: Polymer Chemistry - Wiley Online Library
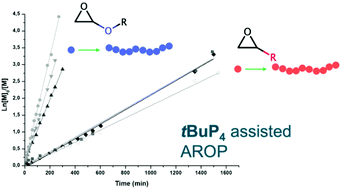
Polymerization of epoxide monomers promoted by tBuP4 phosphazene base: a comparative study of kinetic behavior - Polymer Chemistry (RSC Publishing)

31 P { 1 H} NMR spectra of the pure (a) phosphazene base 2d and (b) PIL... | Download Scientific Diagram

Structural Effect of Organic Catalytic Pairs Based on Chiral Amino(thio)ureas and Phosphazene Bases for the Isoselective Ring-Opening Polymerization of Racemic Lactide | Macromolecules

Two-Step Asymmetric Synthesis of Disubstituted N-Tosyl Aziridines Having 98−100% ee: Use of a Phosphazene Base | The Journal of Organic Chemistry
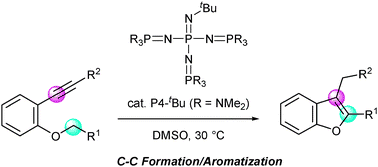
Phosphazene base-catalyzed intramolecular cyclization for efficient synthesis of benzofurans viacarbon–carbon bond formation - Chemical Communications (RSC Publishing)
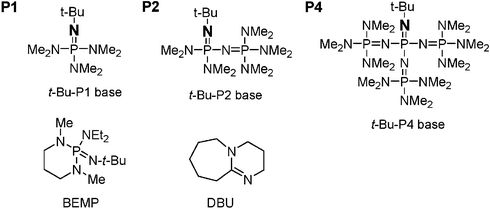
Phosphazene base-catalyzed condensation of trimethylsilylacetate with carbonyl compounds - Chemical Communications (RSC Publishing) DOI:10.1039/B606056K